| Product No. | ST069 |
|---|---|
| CAS Reg. No. | 2135-17-3 |
| Alternate CAS Reg. No. | - |
| Offer | 100 mg |
2135-17-3
- Documentation
- Details
Chemical name
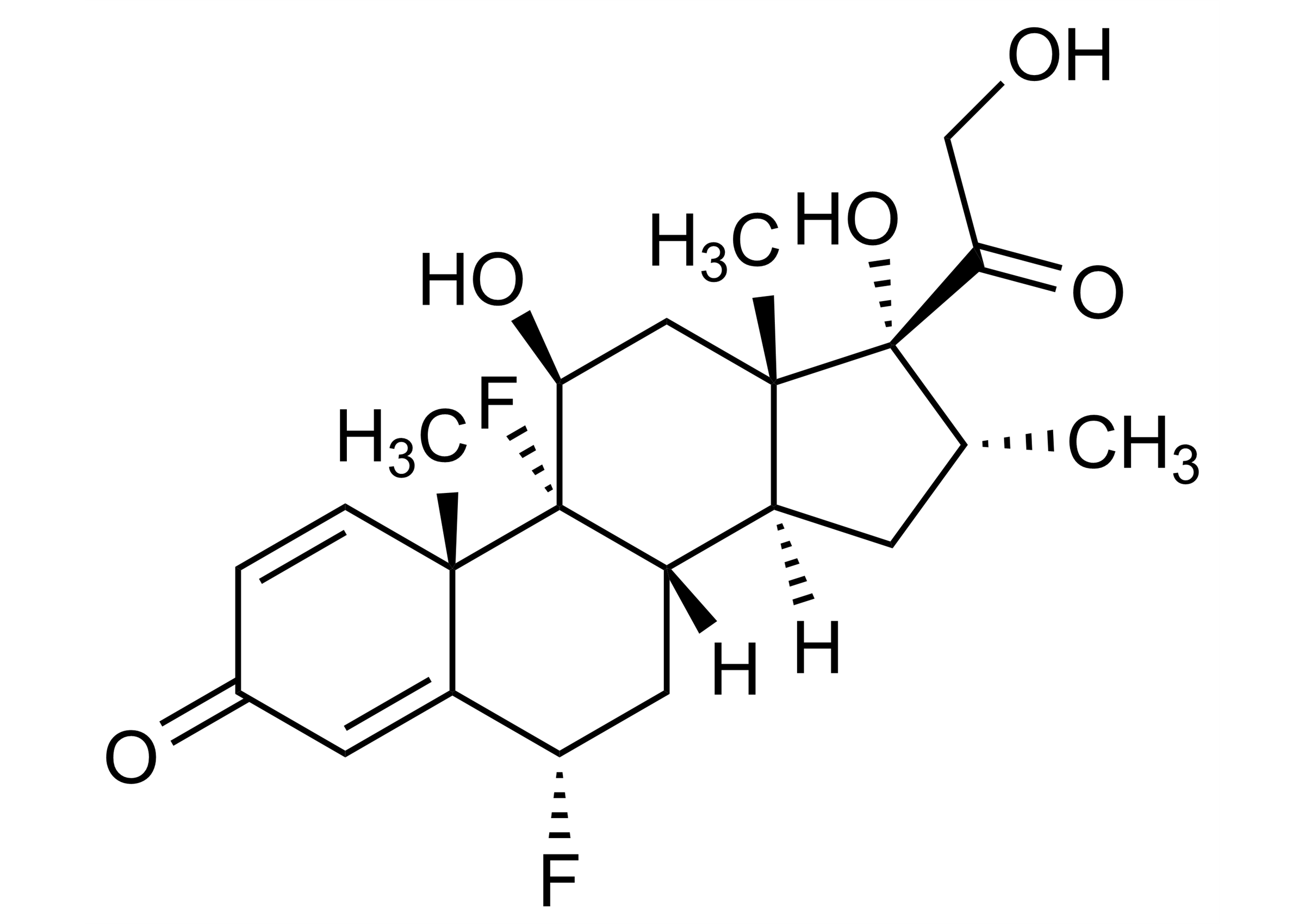
(6α,11β,16α)-6,9-Difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
Description
Flumethasone (CAS 2135-17-3) reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH supports LC-MS/MS and GC-MS quantification. This glucocorticoid reference material enables traceable calibration and robust method validation. As an LC-MS/MS calibration standard and GC-MS confirmation standard, it ensures accuracy and reproducibility from screening to confirmatory analysis.
This reference standard is produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. Each lot is supplied to support traceability in quantitative analysis. Laboratories use it to establish calibration ranges, verify instrument performance, and confirm target identity with orthogonal detection.
Typical applications include the following:
- Regulated laboratories: routine residue analysis and reporting.
- Pharmaceutical research: metabolism studies and metabolite profiling.
- Food and feed testing: residue control and surveillance.
- Method development: multi-residue workflows and recovery checks.
- Confirmatory analysis: second-source verification using GC-MS or LC-MS/MS.
Flumethasone (CAS 2135-17-3) reference standard supports reliable calibration and quality control. Prepare calibration curves and QC samples across relevant levels. Use matrix-matched solutions to assess recovery and matrix effects. The material helps validate precision, accuracy, and linearity. It also supports decision limits in confirmatory analysis. With proper documentation, traceability is maintained throughout the workflow.
For routine work, apply this reference standard in LC-MS/MS or GC-MS to verify retention time, ion ratios, and response factors. It assists method validation steps such as selectivity, robustness, and stability checks. As a result, laboratories can streamline multi-residue method development and strengthen compliance with reporting requirements.
Safety Data Sheet
You can download your Safety Data Sheet for ST069
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | (6α,11β,16α)-6,9-Difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione |
|---|---|
| Molecular Formula | C22H28F2O5 |
| Molecular Weight | 410.45 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | ST069 |
| CAS Reg. No. | 2135-17-3 |
| Alternate CAS Reg. No. | – |
| Offer | 100 mg |